To gain a better understanding of the DOFSs, we need to explore their key components, including the laser, optical fiber, photodiode, and scattering phenomena (Rayleigh, Raman, and Brillouin) in optical fiber one by one. Before we do that, let’s refresh our knowledge of the historical debate about the nature of light and the concept of wave-particle duality.
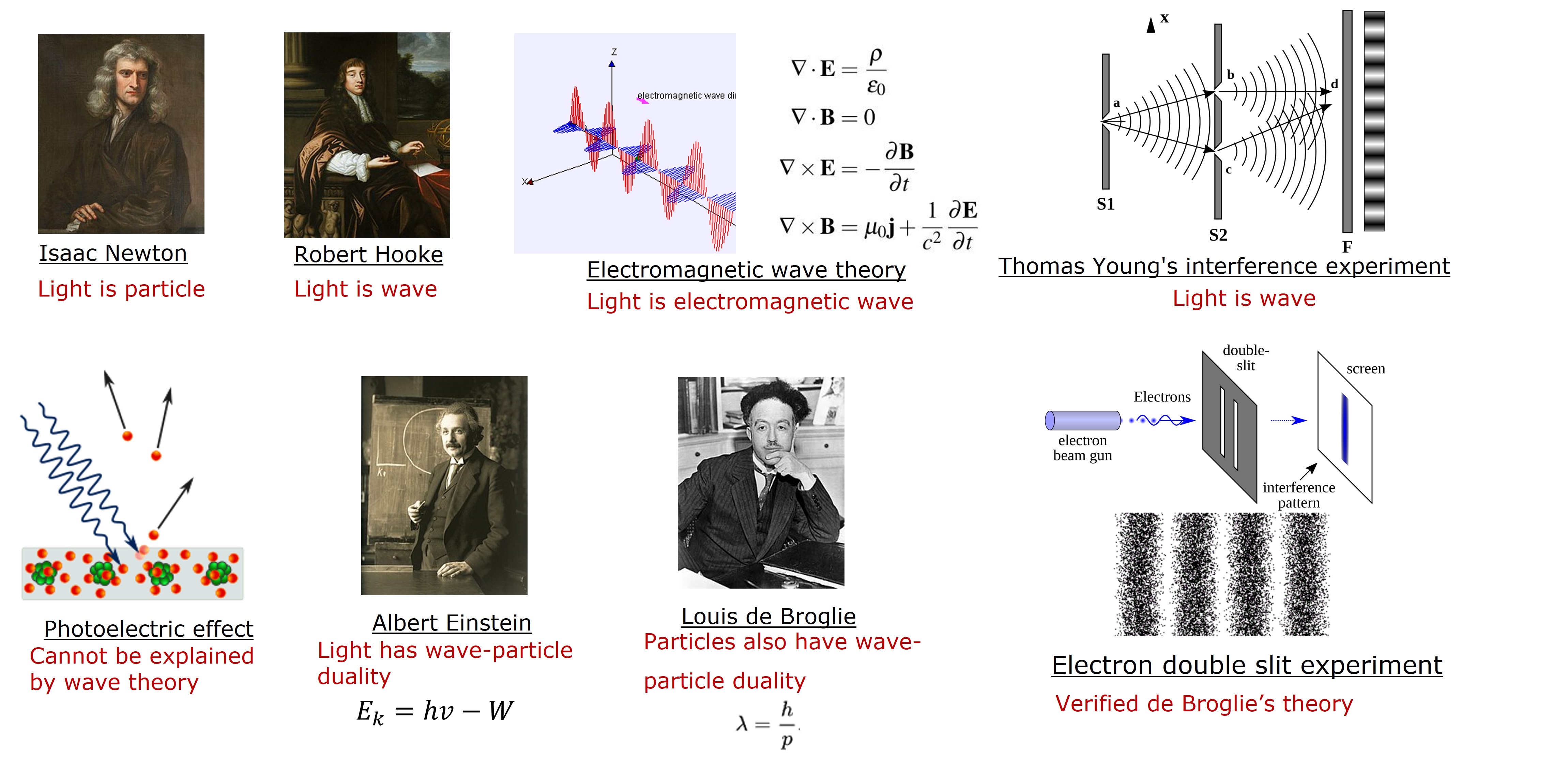
The nature of light and wave-particle duality
The nature of light has been a subject of debate since the 17th century, with renowned physicists like Isaac Newton and Robert Hooke offering contrasting views. Newton considered light as tiny particles, while Hook perceived it as a type of wave. In the 18th century, Maxwell’s Electromagnetic wave theory supported the wave nature of light, specifically an Electromagnetic (EM) wave. Thomas Young’s interference experiment strengthened the wave theory. However, this theory could not explain the photoelectric effect. To explain the phenomenon, Albert Einstein, in 1905, proposed a theory that light has particle behavior when it interacts with material. He used the term ‘photon’ to describe the discrete energy packets of light. Einstein used the equation \((E_k=hv-W)\) to explain the photoelectric effect, where \(E_k\) is the kinetic energy of the ejected electrons, \(W\) is the energy required to remove an electron from the surface of the material, and \(hv\) is light’s energy. Photon relates to its frequency \((v)\), and Planck’s constant \((h)\) and is discrete. This indicates the wave-particle duality of light - frequency \((v)\) suggests the wave behavior and discrete energy packets of light suggest the particle behavior of light. The wave-particle duality laid the foundation for quantum mechanics. De Broglie later expanded this duality to solid particles, such as atoms and electrons, presenting the equation \((\lambda=h/p)\) to calculate their wavelengths. De Broglie’s theory was verified by the electron double-slit experiment and significantly advanced quantum mechanics.
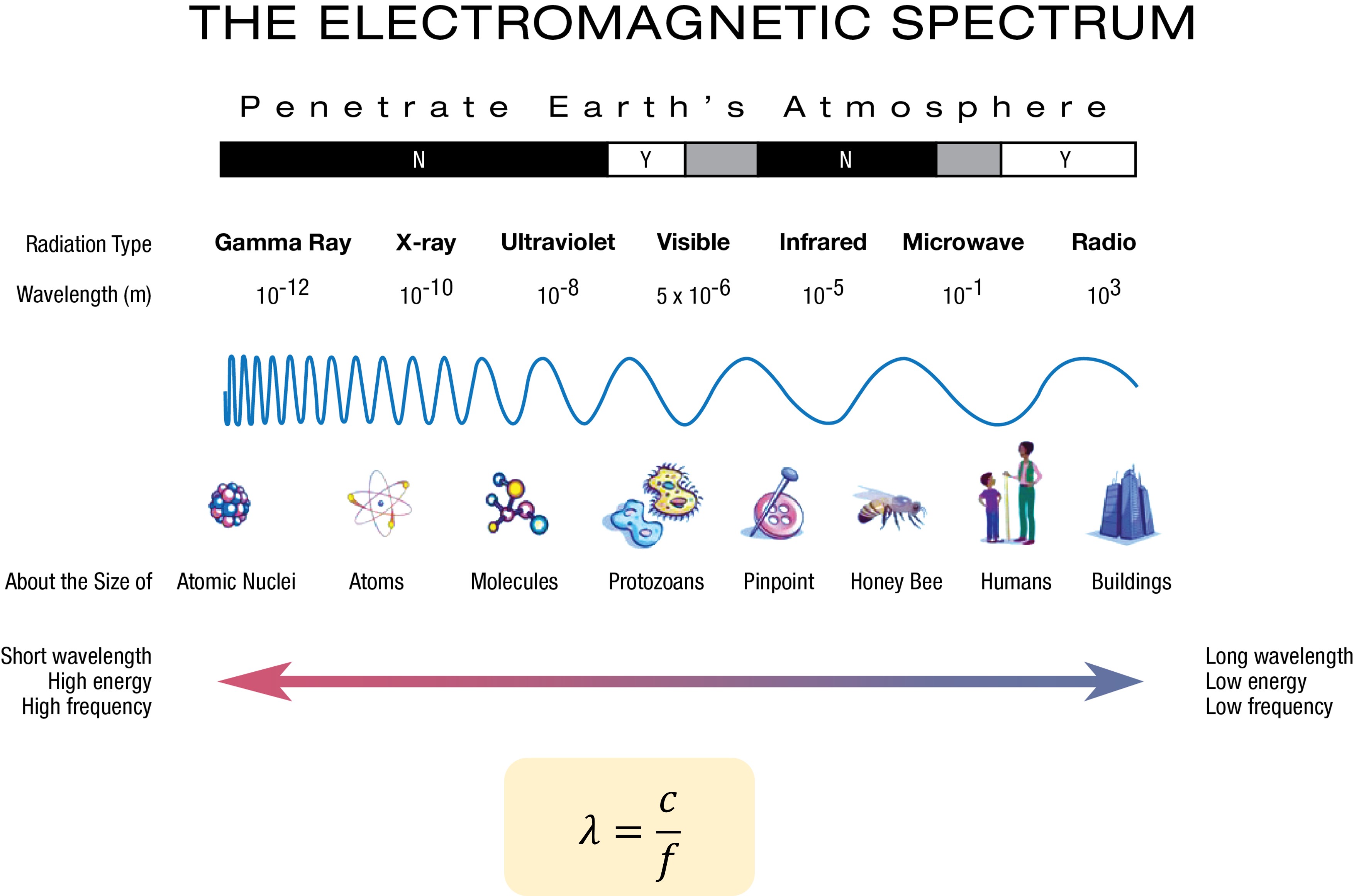
Electromagnetic wave theory
For objects much larger than the light’s wavelength, EM wave theory is still suitable. EM waves are a collection of electrical and magnetic fields, that induce each other. Thus, the propagation of EM waves does not need a medium. Consequently, light and microwaves can traverse outer space. The EM spectrum classifies waves into different bands based on frequency or wavelength, including radio, microwave, infrared, visible, ultraviolet, X-ray, and Gamma-ray. Higher frequency correlates to shorter wavelengths. In describing waves, we often use wavelength, frequency, phase, and polarization to represent their properties.
The atom world
Atoms consist of a nucleus and orbiting electrons in discrete shells, each corresponding to an energy level. Bohr’s model successfully explained the discrete emission and absorption spectrum of hydrogen. However, it was not entirely accurate and difficult to explain the spectra of other elements and the wave behavior of light. Integrating de Broglie’s material wave concept, the electron’s orbiting paths became patterns with perimeters being integer multiples of the electron’s wavelength, as indicated in the following right figure. This model considers both particle and wave behaviors of electrons.
In the forthcoming sections, as we explore DOFSs, we will encounter situations where light is treated as a wave when interacting with objects much larger than the light wavelength. Conversely, we will also encounter cases where both light and the object’s wave-particle nature should be considered when light interacts with objects comparable with light wavelength.
Next Part4
Reference
- The Feynman Lectures on Physics, https://www.feynmanlectures.caltech.edu/
- Orzel C. Breakfast with Einstein: The exotic physics of everyday objects. BenBella Books, 2018.